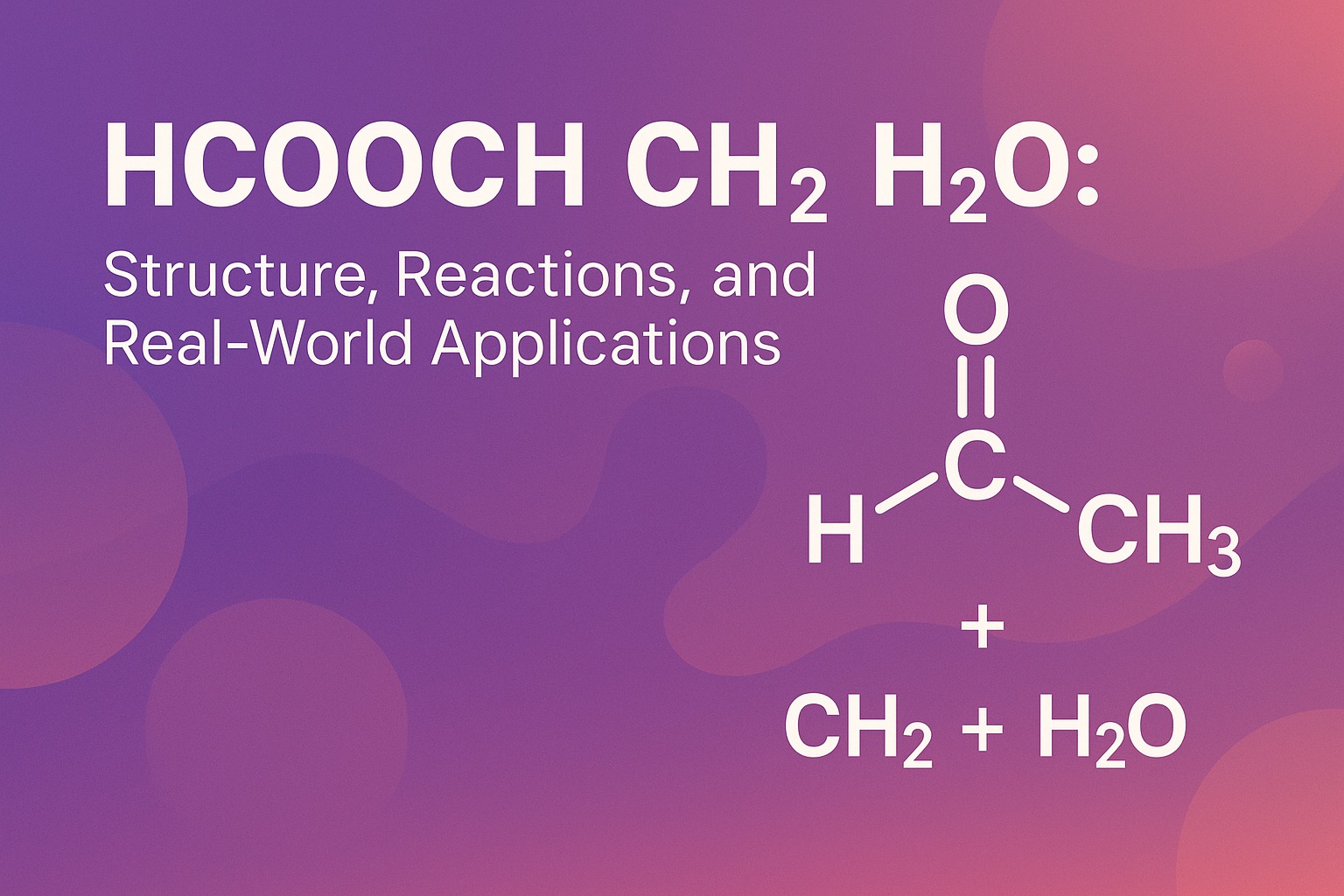
In the intricate dance of molecules that defines our physical world, few reactions are as fundamental and instructive as the hydrolysis of simple esters. The reaction represented by hcooch ch2 h2o serves as a perfect archetype for this process. While this notation is a simplified representation, it typically refers to the hydrolysis of methyl formate, a crucial reaction with significant implications in both industrial chemistry and biological systems. This article delves into the mechanics, applications, and future-forward perspectives of this essential chemical transformation as understood in 2025.
What Exactly is HCOOCH₂ + H₂O?
At its core, hcooch ch2 h2o depicts a hydrolysis reaction. Hydrolysis, derived from Greek words meaning “to break with water,” is a chemical process where a molecule is split into two parts by the addition of a water molecule. Here, hcooch ch2 h2o is the ester methyl formate (HCOOCH₃), and H₂O is water.
The products of this reaction are formic acid (HCOOH) and methanol (CH₃OH). The balanced chemical equation is:
HCOOCH₃ + H₂O → HCOOH + CH₃OH
This transformation doesn’t occur spontaneously at room temperature; it requires a catalyst to proceed at a practical rate, leading us to the critical concept of catalysis.
The Crucial Role of Catalysts in HCOOCH₂ + H₂O
A catalyst is a substance that speeds up a chemical reaction without being consumed itself. For the hydrolysis of methyl formate (HCOOCH₂ + H₂O), there are two primary pathways:
-
Acid-Catalyzed Hydrolysis: Adding a strong acid like sulfuric acid (H₂SO₄) or hydrochloric acid (HCl) provides protons (H⁺ ions) that catalyze the reaction. The acid protonates the carbonyl oxygen of the ester, making it more susceptible to nucleophilic attack by a water molecule.
-
Base-Catalyzed Hydrolysis (Saponification): Using a strong base like sodium hydroxide (NaOH) also catalyzes the reaction. The base deprotonates the water molecule, creating a more powerful nucleophile (OH⁻ ion) that attacks the ester’s carbonyl carbon. In this case, the products are the formate ion (HCOO⁻) and methanol, with the formate ion later being converted to formic acid.
The choice between acid or base catalysis depends on the desired products and the specific industrial process.
Industrial Applications of hcooch ch2 h2o Hydrolysis
The reaction hcooch ch2 h2o is not merely a textbook example; it is a workhorse in chemical manufacturing. Its primary industrial application is the production of formic acid.
-
Formic Acid Production: Formic acid is a valuable commodity chemical used as a preservative, antibacterial agent, and coagulant in the rubber and textile industries. The most common industrial method for producing pure formic acid involves a two-step process where carbon monoxide and methanol first react to form methyl formate hcooch ch2 h2o. This ester is then hydrolyzed using the HCOOCH₂ + H₂O reaction under catalytic conditions to yield formic acid and methanol, which is often recycled.
This efficient process highlights the reaction’s importance in sustainable chemical synthesis, minimizing waste and maximizing resource utilization.
The Biological Connection: Enzymatic Hydrolysis
The principle behind hcooch ch2 h2o is mirrored endlessly in biology. While methyl formate itself is not a common biological molecule, the hydrolysis of esters is a universal reaction. Our bodies use sophisticated biological catalysts called enzymes to perform hydrolysis with incredible specificity and speed.
For instance, the digestion of dietary fats involves enzymes like lipase breaking down triglyceride esters (fats) through hydrolysis—a reaction analogous to hcooch ch2 h2o but on a more complex molecule. This biological parallel underscores the fundamental nature of hydrolysis in life processes.
Future Outlook and Environmental Considerations
As we move further into the decade, the principles governing hcooch ch2 h2o are being refined for a greener future. Research in 2025 focuses on developing more efficient and environmentally benign catalysts, such as:
-
Solid Acid Catalysts: Replacing liquid mineral acids with solid acids to simplify product separation, reduce corrosion, and minimize hazardous waste.
-
Enzyme-Mimetic Catalysts: Designing synthetic catalysts that operate with the high efficiency and selectivity of natural enzymes, potentially allowing for hydrolysis under milder temperature and pressure conditions.
These advancements aim to make industrial processes reliant on reactions like hcooch ch2 h2o more sustainable and energy-efficient, aligning with global green chemistry initiatives.
Deep Dive: The Mechanism of HCOOCH₂ + H₂O
To truly appreciate the HCOOCH₂ + H₂O reaction, one must understand its step-by-step mechanism. Let’s explore the acid-catalyzed pathway, which is a classic example of nucleophilic acyl substitution.
-
Activation (Protonation): The carbonyl oxygen of the methyl formate ester (HCOOCH₃) is protonated by the acid catalyst (e.g., H₃O⁺). This crucial first step adds a positive charge to the molecule, making the central carbonyl carbon far more electrophilic (attractive to nucleophiles).
-
Nucleophilic Attack: A water molecule, acting as a nucleophile, attacks this now highly electrophilic carbonyl carbon. This step forms a tetrahedral intermediate—a temporary structure where the carbon is bonded to four separate atoms.
-
Proton Transfer: The intermediate undergoes proton transfers to yield a viable leaving group.
-
Elimination: The methanol molecule (CH₃OH) is eliminated from the intermediate, resulting in a formic acid molecule (HCOOH) and regenerating the acid catalyst (H⁺), which can then catalyze another reaction.
This elegant, multi-step dance explains how and why the reaction proceeds, transforming the neutral ester into carboxylic acid and alcohol products.
Optimizing the HCOOCH₂ + H₂O Reaction in Industry
In an industrial setting, simply mixing reactants is not enough. Efficiency, yield, and cost are paramount. For the HCOOCH₂ + H₂O reaction, this involves careful engineering of reaction conditions.
-
Temperature and Pressure: The hydrolysis is typically conducted under elevated temperatures (often between 50-80 °C) and sometimes increased pressure. This provides the reactant molecules with more kinetic energy, increasing the frequency of successful collisions and speeding up the reaction rate significantly.
-
Reactant Ratios: Often, an excess of water (H₂O) is used. According to Le Chatelier’s Principle, this shifts the equilibrium position of the reversible reaction to the right, favoring the formation of more formic acid (HCOOH) and methanol (CH₃OH), thereby maximizing the yield of the desired products.
-
Catalyst Recovery: A major focus in 2025 is on catalyst design that allows for easy separation and reuse. For base catalysis, this might involve fixed-bed reactors with solid base catalysts. For acid catalysis, moving away from corrosive liquid acids to solid acid resins (e.g., ion-exchange resins) is a key industry trend, reducing equipment corrosion and environmental waste.
Beyond Formic Acid: The Versatility of Ester Hydrolysis
While our focus is on HCOOCH₂ + H₂O, it’s vital to recognize that this reaction is a single example of a vastly important reaction class. The hydrolysis of esters is a universal tool.
-
Soap Manufacturing: The base-catalyzed hydrolysis of fatty acid triglycerides (from plant oils or animal fats) is called saponification. This reaction produces glycerol and soap molecules (carboxylate salts), a process humanity has relied on for centuries.
-
Polymer Degradation: Biodegradable plastics, such as polylactic acid (PLA), break down in the environment through hydrolysis. The ester linkages in the polymer chain are attacked by water, eventually breaking the material down into harmless, natural metabolites. Understanding reactions like HCOOCH₂ + H₂O is fundamental to designing the next generation of sustainable materials.
-
Pharmaceutical Synthesis: Many drug molecules are esters or contain ester functional groups. Controlled hydrolysis is a key step in both synthesizing these active pharmaceutical ingredients (APIs) and understanding how they are metabolized in the body by enzymes like esterases.
Conclusion
The deceptively simple equation hcooch ch2 h2o represents a gateway to understanding a reaction of profound importance. From its role as a classic example of hydrolysis in organic chemistry to its critical function in large-scale formic acid production and its echoes in human biology, this reaction is a cornerstone of molecular science. As catalytic technology advances, the ongoing optimization of this process ensures that
In the intricate dance of molecules that defines our physical world, few reactions are as fundamental and instructive as the hydrolysis of simple esters. The reaction represented by hcooch ch2 h2o serves as a perfect archetype for this process. While this notation is a simplified representation, it typically refers to the hydrolysis of methyl formate, a crucial reaction with significant implications in both industrial chemistry and biological systems. This article delves into the mechanics, applications, and future-forward perspectives of this essential chemical transformation as understood in 2025.
Final verdict: A Reaction Connecting Past and Future
The equation HCOOCH₂ + H₂O is a powerful symbol in chemistry. It connects the foundational principles of organic reaction mechanisms taught in classrooms to multi-ton industrial processes that produce essential chemicals. It mirrors the very biological processes that sustain life through enzymatic action. As we advance through the 21st century, the lessons learned from optimizing this specific reaction are directly applicable to the grand challenges of green chemistry, sustainable manufacturing, and environmental remediation. The continued study and refinement of the HCOOCH₂ + H₂O hydrolysis ensure that this classic chemical transformation will remain deeply relevant, proving that even the simplest-looking reactions can hold the key to profound scientific and industrial progress.